Suggestions for Microwire Insertion
General Procedures
The following are general suggestions for insertion of TDT microwire arrays and may not comply with your animal care and use guidelines. Investigators should consult officials at their respective institutions to determine the regulations governing animal care and use in their laboratory.
We use aseptic techniques and avertin anesthesia for mouse, ketamine/xylazine anesthesia for rat.
We use the general procedures for rodent survival surgery described in: "Principles of Aseptic Rodent Survival Surgery: General Training in Rodent Survival Surgery - Part I" In: Laboratory Animal Medicine and Management, Reuter J.D. and Suckow M.A. (Eds.) International Veterinary Information Service, Ithaca NY, 2004; B2514.0604.
This can be viewed at https://www.ivis.org/library/laboratory-animal-medicine-and-management/principles-of-aseptic-rodent-survival-surgery.
NIH offers instructional videos entitled: "Training in Basic Biometholodology for Laboratory Mice" and "Training in Survival Rodent Surgery" at their website: https://olaw.nih.gov/education/training-videos.htm.
Stereotaxic Surgery
We use procedures similar to those described in: "Stereotaxic Surgery In The Rat: A Photographic Series" by Richard K. Cooley and C.H. Vanderwolf.
Microwire Procedures
General information, pictures, and available configurations for TDT microwire arrays can be found at:
https://www.tdt.com/component/omnetics-based-electrodes/
https://www.tdt.com/component/zif-clip-array-electrodes/
A paper by Kralik et al. (2001) contains a very helpful description of microwire array insertion methods (Methods. 2001 Oct; 25(2): 121-50).
In rat and mouse, we recommend following the general and neurosurgical procedures as described in the references above.
We first prepare the subject and perform a craniotomy above the implantation site following the methods of Cooley and Vanderwolf (2004). Implant several skull screws as described in this reference to help bond the dental acrylic and array to the skull. A base coat of OptiBond FL (Kerr) applied to the skull works well to help bond the dental acrylic. Keep this out of the craniotomy.
For rat and mouse we recommend a durotomy, using the tip of a sterile syringe needle as a micro-scalpel to cut an "X" shaped incision through the dura. Reflect the flaps of dura aside, taking care not to disturb the pia or pial vasculature.
Advance the array to the pial surface using a stereotaxy and check that all electrodes are unobstructed by bone or dura. We have also used the stereotaxy to quickly advance the array through the pia and then to adjust the array to its final depth. This method has worked well for a number of our customers as well.
There have been two schools of thought on insertion speed. Fast insertion (e.g. Rousche PJ, Normann RA. Ann Biomed Eng. 1992;20(4):413-22) using an inserter device, and slow insertion (e.g. Nicolelis et al., Proc Natl Acad Sci U S A. 2003 Sep 16;100(19): 11041-6). A paper by Rennaker et al., 2004, (J Neurosci Methods. 2005 Mar 30;142(2):169-76) explores the relative merits of each method.
Regardless of which insertion method you choose, advance the array to its desired position, leaving it attached to the stereotaxy until it is fully bonded to the skull with dental acrylic. Prevent CSF from weeping from the craniotomy by gently packing around the array with gelfoam. The CSF will eventually soak through and keep the acrylic around the craniotomy from curing, so perform this step quickly. Bone wax or Kwik-Cast would probably work better than the gelfoam, but we have not used these in our lab to date.
Attach the array to the skull using a thin layer of dental acrylic and the methods described by Cooley and Vanderwolf. Do not build up a large base of acrylic until the ground wire(s) of the array have been attached by wrapping them around the stainless skull screws. Make very sure that the ground wire(s) make good electrical contact to the screws. Pot the entire array/screw complex with dental acrylic using the methods described by Cooley and Vanderwolf.
In our hands, explanted arrays come out of the brain with roughly the same impedance they went in with. Here, recording duration seems to be more limited by surgical technique/capsule formation than by the arrays themselves. We recommend ethylene oxide gas sterilization of the arrays and good sterile surgical technique.
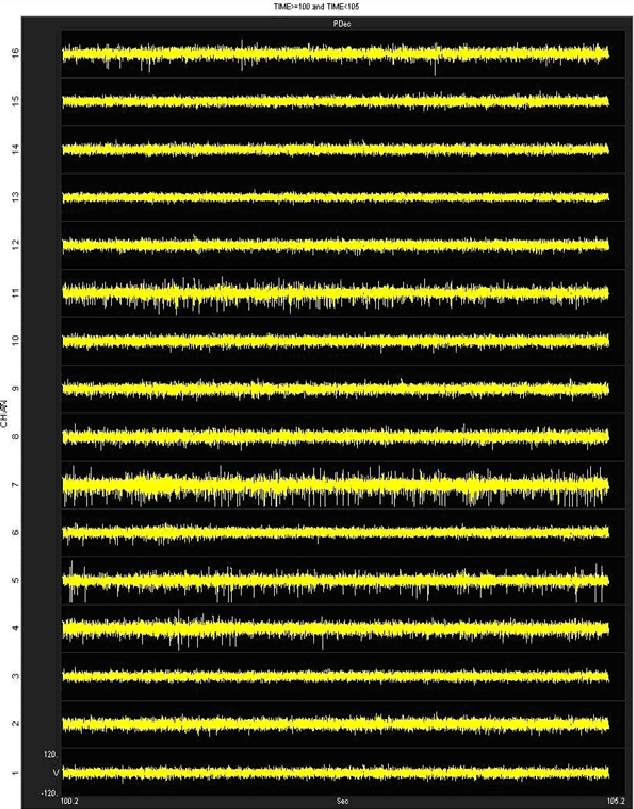
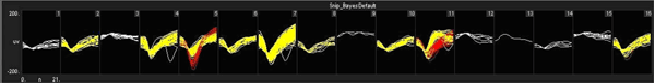
We have obtained good recordings in rat and mouse cortex for several weeks; using only alcohol sterilization of the arrays (we have no access to ethylene oxide). An example from rat with lots of active channels, ~150 uV spikes on ~20 uV background noise is below. We have seen up to ~300 uV spikes on the same noise floor. Our customers have reported recordings durations of several months in rat and monkey.